[ad_1]
The Meals and Drug Administration (FDA) has cleared the Mosie Child Package for over-the-counter (OTC) residence use by people who’ve been unable to conceive via intercourse or select to not conceive via intercourse, for semen assortment and the supply of semen or donor sperm to the vaginal canal.
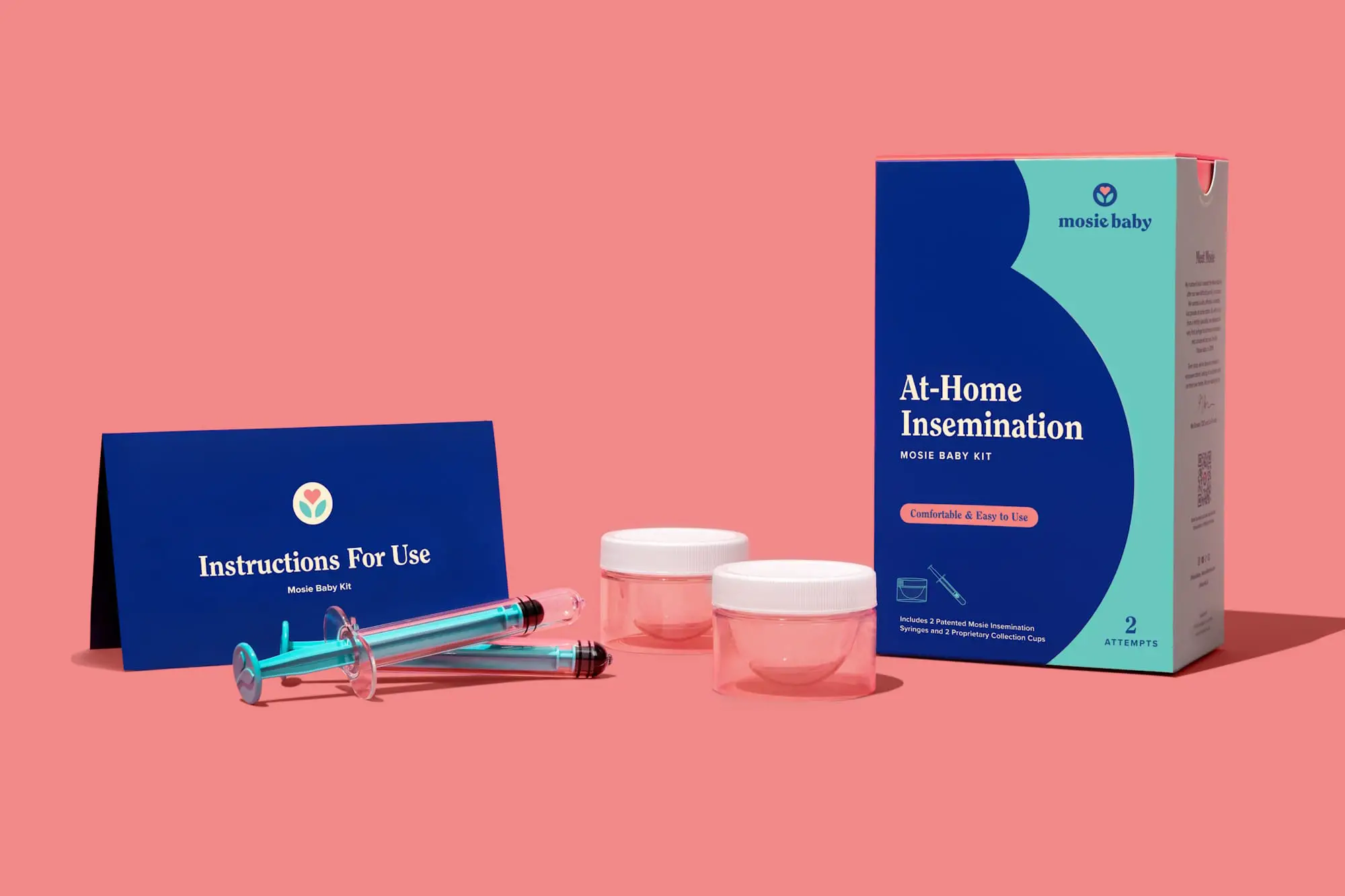
The Mosie Child Package consists of 2 proprietary assortment cups and syringes, used to withdraw the semen from the gathering cup and deploy the semen intravaginally. The system needs to be used in the course of the ovulatory section of the menstrual cycle.
The FDA clearance was supported by scientific information (ClinicalTrials.gov Identifier: NCT04646291) that demonstrated individuals had been in a position to determine what the package was meant for and whether or not it was acceptable for them to make use of the product. Outcomes from scientific research additionally confirmed individuals had been in a position to perceive the instructions on the system labeling. Different assessments included a human sperm survival assay, vaginal irritation testing, and biocompatibility testing. Based mostly on these findings, the Mosie Child Package was decided to be considerably equal to a predicate system utilized in clinically administered intrauterine insemination.
Proceed Studying
“Since inventing the Mosie Child Package in 2014, we realized we weren’t alone in our fertility journey because it’s reported that one in six folks expertise infertility,” mentioned co-founder and CEO Maureen Brown. “So far, we’re very proud to share that Mosie Child has helped greater than 100,000 households inseminate from the consolation of their very own residence. We at the moment are thrilled to supply our system as an FDA reviewed possibility for households trying to inseminate at residence.”
The Mosie Child At-Residence Insemination Package is on the market for $129.99 at mosiebaby.com, CVS.com (or CVS shops), Walmart.com and optumstore.com. The package is anticipated to launch in extra shops in 2024, in keeping with the Firm.
Reference
- Mosie Child turns into the primary firm to obtain FDA clearance for at-home intravaginal insemination. Information launch. Mosie Child. December 6, 2023.
- US Meals and Drug Administration. Mosie Child Package. 510(ok) Abstract. Accessed December 7, 2023.
This text initially appeared on MPR
Need to view extra content material from Medical Advisor? Register now at no cost to entry limitless scientific information with customized every day picks for you, full-length options, case research, convention protection, and extra. {{login-button}} {{register-button}} Please login or register first to view this content material.
Need to learn extra?
[ad_2]
Source link